Ian Sanders wants to feed the world. A soft-spoken Brit, Sanders studies fungus genetics in a lab at the University of Lausanne in Switzerland. But fear not, he’s not on a mad-scientist quest to get the world to eat protein pastes made from ground-up fungi. Still, he believes—he’s sure—that these microbes will be critical to meeting the world’s future food needs.
Sanders’s eyes widen with delight and almost childlike glee when he talks about a microscopic life form called mycorrhizal fungus, his chosen lifetime research subject. Mycorrhizal fungi live in a tightly wound, mutually beneficial embrace with most plants on the planet. Years of dedication have made Sanders into one of the world’s foremost experts on the genetics of the microbe, and he recently was part of a team that sequenced the first mycorrhizal fungi genome.

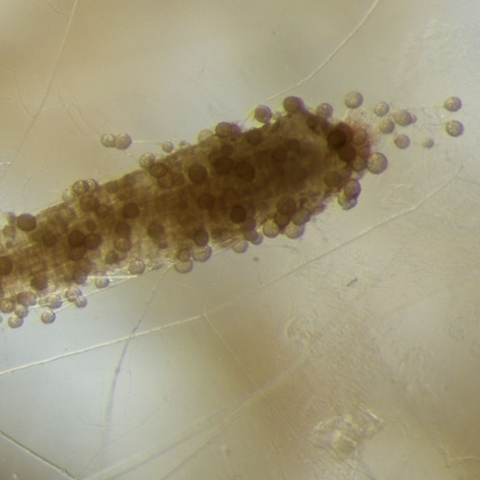
^ Mycorrhizal fungi colonize the tip of a root, seen here under magnification.
Despite his drive, Sanders comes across as light-hearted as he teases and jokes with fellow researchers. But he loses his affable smile as he fires off facts about the upcoming food shortage: The world’s population is expected to increase to between 9 billion and 16 billion people. Five million people per year die of direct causes of malnutrition. Three and a half million of those are children under five. Today, we have the means to grow enough food to feed all those people, but we will most certainly need to produce more in the very near future.
Sanders may have come up with a way to do just that. He has successfully bred custom varieties of microbes that can help plants produce more food. It’s one of the ultimate goals of farming research—more food with, he hopes, little or no environmental downside.
The question of crop productivity is increasingly fraught. People in developed countries eat an enormous amount of food, and people in developing countries are beginning to close the gap. Meanwhile, the world’s population is swelling. By 2030, the UN’s Food and Agriculture Organization predicts food demand will soar by 35%. And then there’s the accelerating impact of climate change: The IPCC’s latest report on the subject, published in March, shows that scientists are predicting a 2% decrease in crop yields per decade over the next century. Higher temperatures and longer, more dramatic swings between drought and rain mean the plants that we rely on will have a hard time weathering the strain.
According to the FAO, most of the growth in production that we’ll need has to come from increasing yields from crop plants. Selective breeding doesn’t seem to be offering the types of dramatic yield increases seen in the past. Meanwhile, genetic engineering has yet to lead to any significant increase in yields.
Now, many scientists are saying that we’ve been looking at the wrong set of genes. Instead of in plants, the crucial genes may reside in the galaxy of bacteria and fungi that live in the soil and throughout a plant—the kind that Sanders studies.
Sanders’ plan is to give existing fungi-plant relationships a boost by breeding better fungi. He’s testing varieties of lab-grown microbes out in the field in tropical Colombia. There, he’s hoping to help cassava plants grow heftier roots, as these potato-like crops are a staple for nearly a billion people around the world. So far, the results show that this approach just might work.
Belowground Microbiome
Microbes in the soil function much like the human microbiome, which helps us break down food, access nutrients, and defend against harmful invaders. A plant’s microbiome protects it against malevolent microbes. Microbes can also communicate with one another, flashing chemical alerts that let one plant know when another nearby is under attack. Bacteria and fungi even structure the soil so that it clumps together and doesn’t blow or wash away. And, just as our human cells are outnumbered by our microbial support, the microbial genes in and near the root system alone of a healthy plant greatly eclipse those of the plant itself.
Plants have depended on microbial assistance since they first edged out of the water onto dry land, about 450 million years ago. They lassoed photosynthetic cyanobacteria and turned them into cellular machines known as chloroplasts, which harvest the sun’s energy. Today, plants are still supported by hundreds of thousands—perhaps millions—of different species of bacteria, fungi, even viruses. In fact, the rhizosphere, the area around a plant’s roots, is considered one of the most ecologically diverse regions on the planet.
The microbiome in the rhizosphere acts as an extension of plants’ root systems, breaking down nutrients into forms that plants can use. Mycorrhizal fungi have whisper-thin fronds, called hyphae, that reach out past the root tips to access water and nutrients the plant needs to survive. They then trade those for carbohydrates the plant provides. Scientists believe that as much as 30% of the carbon that a plant produces through photosynthesis is pushed into the soil to support an entire city of microbes.
Though mycorrhizal fungi are just a multitude microbe species in the soil in and around plant roots, they live in symbiosis with about 80–90% of agricultural crops in a relationship hundreds of millions of years old. Mycorrhizal fungi cannot survive without plants, and most plants cannot thrive without mycorrhizal fungi.
On the most basic level, scientists have known that microbes associate with plants for more than a century, but, even today, many of the details of the interactions are still unknown. Part of the challenge in teasing them out is that they’ve been nearly impossible to study. Scientists estimate that perhaps 1% of all soil microbes can be grown on a petri dish, the conventional model for such research. By only being able to study the thinnest slice of life, we’ve been missing out on a vast, complicated, messy world. It’s like trying to guess what everyone on a city block does during the day by trailing just one person.
Recently, though, scientists have begun to get a better glimpse. Genetic analyses can help classify and understand newly discovered microbes. Big Data-style techniques, with names like metagenomics, proteomics, and transcriptomics, describe methods by which scientists can take an overall picture of the genetic diversity of life in a given region, and even what genes are active. These types of studies might not be able to describe every individual, but they can give a sense of what genes are in play. Such tools are able to do more, do it more quickly, and do it for less money nearly every year.
In only the last few years, scientists using these tools have begun to regularly uncover new information about the crucial links between microbes and plants. They’re unraveling clues as to which bacteria, fungus, or virus performs which function. They’re discovering microbes that can help plants withstand heat and drought. And they’re dialing into the genetics to understand how the microbes do what they do, how the plants react, and even what genetic material is exchanged. There’s still a world of research to be done, however. With many millions of individuals packed into every gram of soil, it’s a daunting task.

^ Tending a cassava field in the Amazon
“Over the last hundred years in agriculture, we’ve tried to take microorganisms out of the picture. And by doing that—by disrupting the soil with tillage, by using chemical pesticides—we have greatly altered the agricultural biome,” says Rusty Rodriguez, a former microbiologist with the U.S. Geological Survey who’s now head of Adaptive Symbiotic Technologies, a company developing microbial-based seed coatings. “The efficacy of many chemicals is beginning to wane.” Bacteria and fungi, Rodriguez says, “are the next paradigm for agriculture.”
From Switzerland to Columbia
Sanders’ Swiss workplace is immaculately clean, and the room where the fungi are taken out for study is scrupulously sterile. Every night, all night, UV lights shine a microbe-killing glare. They destroy anything that could infect his cultures of mycorrhizal fungi.
Over the course of Sanders’ 26-year career, he’s made a number of key discoveries about fungi genetics and reproduction. He conducted early research that demonstrated that the greater the diversity of mycorrhizal fungi in a given ecosystem, the greater the diversity of plants. And in 2008, as he delved into genetics, he proved that they don’t just reproduce by cloning—they actually exchange genetic material, both in the lab and in the field.
This gave him an idea. If the microbes created offspring that were different from one another, Sanders thought, “you have a good chance that some will be more effective on plant growth than others.” So he came up with a plan: Take different fungi, breed them, see if any help plants out more than others. In other words, take the approach to farming that breeders have used for thousands of years and use it on fungi.
This is where Sanders runs into occasional criticism from some of his microbe-studying colleagues, who say that nature has already bred all the best variety of microbes. “If you use the argument from these researchers,” he counters, “then no one would have produced any plants through plant breeding, because they would have said, ‘Well, nature’s already made the best plants, and we can’t make any more that are any better than what nature has made.’ Now, of course, we know from a few thousand years of agriculture that we can make plants better by crossing them, and we can get varieties that produce bigger yields than that which we see in natural-occurring varieties of those plants in nature.” Without similar human intervention, the whole system of microbial support might not be optimally tweaked to match.
To test out his idea, Sanders partnered with a colleague in Switzerland who was studying the genetics of the fungi-rice relationship, and who already had conducted research in a university greenhouse set up for rice cultivation. Sanders grew the fungi and allowed them to exchange genetic material and reproduce, creating genetically distinct offspring. Then, he colonized rice with these distinct lines. Sanders used rice as a matter of convenience due to his colleague’s experience, but he also knew that rice, as farmed today, tends to actually grow more poorly when inoculated with mycorrhizal fungi, making it a good test bed. He was stunned when one of the lines produced a five-fold increase in growth over the other fungal lines. “To see such a huge growth increase was very, very surprising,” he says. The greenhouse was an artificial environment, and the microbe-enhanced soil was compared to sterile soil. It in no way mimicked nature. But it proved a point.
Around that time, Sanders got back in touch with Alia Rodriguez, an agronomist in Colombia who also had expertise in mycorrhizal fungi. They had originally met when he was one of her PhD examiners in England. He was desperate to visit Colombia and see its amazing animal and plant biodiversity for himself, so they decided to try to find a research project together.
It happened that Colombia offered the perfect field test for his new approach. Mycorrhizal fungi are skilled at helping plants access phosphorus, a key nutrient, which plants in tropical countries have a particular problem securing. The acidity of soil there results in a chemical reaction that ties up most of the phosphate that farmers add to soil. Farmers end up paying precious money to add phosphate that plants mostly can’t use. “I always tell my students, how can we rely on a practice that is so inefficient?” Rodriguez says. “It has to change, because it cannot be sustainable.”

^ Ian Sanders and Alia Rodriguez's experimental plots in Columbia
Colombia is also the home of cassava, a fleshy white root. Cassava is a major staple for nearly a billion people in more than 100 countries, from Brazil to Nigeria to Thailand, who rely on it in much the same way we rely on bread or potatoes. In its various homes and in various languages, it is called cassava, yuca, manioc, balinghoy, kamoteng kahoy, tapoica-root. If you can produce more cassava, then poor communities can eat more food.
Sanders liked the idea of breeding microbes to increase cassava production. But they still had one major stumbling block ahead. There was no practical way to transport enough pure fungus from his Swiss lab to colonize the cassava trial fields in Colombia.
This had also been a problem for the early pioneers in the field. In earlier decades, a variety of start-ups had marketed mycorrhizal fungi transported in soil, an imperfect medium that also contained plant roots and a host of other microbes. There was no way to tell whether it contained any live, viable material, let alone a specific species. Plus, transporting enough soil for every plant root on a farm would be heavy and prohibitively expensive.
Fortunately for Sanders and Rodriguez, a company in Spain named Mycovitro coincidentally announced the culmination of decades of research of their own: a gel that could act as a vehicle for highly concentrated, purified mycorrhizal fungi. With the gel, Sanders would know that he was only transporting the microbes he wanted. A single small bottle could provide enough fungi for an entire field. Even more importantly, the gel base was capable of growing any variation that Sanders bred in his lab. The team partnered with Mycovitro to grow Sanders’ varieties. (The company has no financial connection to Sanders’ and Rodriguez’s research, and neither of the scientists have a stake in the company. The company, however, is providing its services for free, and it will have first right of refusal to commercialize any promising new line that Sanders and Rodriguez develop.)
With the final piece in place, Sanders and Rodriguez set their research project in motion. They headed down to Columbia to test their varieties by growing hectares of cassava along the edge of the llanos, the country’s lush, damp tropical savannah.
Catching On
As the pieces of Sanders and Rodriguez’ research fell into place, the field of commercially-applicable bio products was undergoing a renaissance. A few decades ago, interest in microbes and their use in agriculture flared, but most of the commercial products quickly flickered out. Most of the laboratory successes hadn’t translated to the field. One of the few agricultural microbes that did catch hold was the bacterium Rhizobium, which helps legumes access nitrogen. It’s used extensively on crops such as soy. Other microbes, such as the bacterium Bacillus, are used to protect plants from pathogens. Rhizobium and Bacillus are not the only examples on the market, but the combined market share is still a small fraction of the multibillion dollar agro-chemical industry.
But new, more effective products have begun to emerge. Marrone Bio Innovations’ most recent pesticide, called Grandevo, was developed from a soil bacterium and is marketed to protect vegetable crops from sucking insects and mites. The company, with more than 150 patents pending, has additional products in the pipeline, including a strain of Bacillus that both controls pathogens and encourages plant growth.
Rusty Rodriguez (no relation to Alia Rodriguez in Colombia), the head of Adaptive Symbiotic Technologies, got his start in the 1990s when he and his colleagues discovered the symbiosis between plants and fungi in Yellowstone that allowed plants to survive in temperatures as high as 150˚ F. Once he identified and isolated the fungus responsible for the plant’s heat-survival ability, he realized he could use it to help other plants survive extreme heat.
Rodriguez dove headfirst into extremophiles, sending company employees to collect plants from extreme environments around the U.S. He’s focusing on a number of products—some are single fungi, others are communities working together—that confer a variety of benefits to agricultural plants: drought tolerance, salt tolerance, and the ability to withstand swings in temperature. His company has developed tests that rule out any potential negative impacts of the strains, such as plant damage or toxicity to animals that might snack on them. They have dozens of field trials in place in 14 states around the U.S., working with farmers who are testing their products in corn, soybeans, wheat, barley, and rice.
Farmers have been willing partners, Rodriguez says, happy to test products that might help what can be a razor thin profit margin. But, overall, the science of applying microbial products in agriculture has been hampered by one major challenge: moving from the lab to the field. “Field work is a lot more difficult to do,” says Rodriguez. “It fails way more often.”
Sanders and Alia Rodriguez learned the same lesson in Colombia, when the floods came.
To the llanos
In Columbia, Sanders and Alia Rodriguez teamed up with an agricultural college named, appropriately, they hoped, Utopia. The professors and students served as field monitors for the crops and the research. Early one morning last July, the sun barely lifting off the flat green fields, I accompanied them and a group of students as they tromped out to visit their plants. Rodriguez poked fun at Sanders’ obsession with snapping photos: “We need to be moving on!” she nudged. “Yes, yes,” he muttered, bending down to focus his lens on a spider whose web spread across the spiny leaves of a pineapple plant.

^ A graduate student tends cassava in an experimental plot.
Finally we reached the experiment. The cassava looked nearly identical, all about three feet tall, creating a waist-high carpet of broad emerald leaves glittering with droplets misted from the low, grey sky. Despite the plants’ near uniform appearance, Sanders and Rodriguez knew that underground, where the fungi were going to work, the story would be different. There, they had expected to find roots of all sizes.
The two scientists wandered out, half obscured by foliage: Rodriguez, with tight, dark ringlets woven into a long, single braid and tucked through the back of a salmon-colored baseball cap, and Sanders, whose pale skin clearly marked him as the outsider in the group. Isabel Ceballos, the Colombian PhD student managing the project, pulled a bright pink poncho over her head to ward off the rain.
Each of the young cassava plants had started out as six-inch sticks. The team had laid them in the earth and covered them with a shallow layer of soil. Three weeks later, when the sticks started to form root buds, the students returned and carefully squeezed a layer of fungus-filled gel beneath a portion of each plant. As the roots stretched into the soil, they pushed down through the gel, inoculating them with mycorrhizae.
That July day in Colombia, after checking the plants in the field, Sanders, Rodriguez, and I dragged plastic chairs together. They’d cleaned up from the morning’s mud. Rodriguez had changed into a striped cotton top, and her hair cascaded in waves over to the side, revealing beaded lime green and black earrings in the shape of lizards. Sanders’ short-sleeve plaid shirt looked clean and fresh. The sun set over Utopia’s low, red-roofed buildings, and the shrill blur of insects tussled with the frogs’ boggy croaks. The air was thick and warm. Fireflies flashed languidly, slow pulses of glowing and dimming light.
“It was a good surprise to see the experiments up and running in the field now,” says Rodriguez, relaxing into the chair. “It’s been a process to get things going here. Finally to see it happening—it’s difficult, but it’s achievable. A good feeling.”
Early on, the team had learned that Mycovitro’s own variety of mycorrhizal fungi increased cassava yields by as much as 20%. Now their own custom, lab-grown microbes were being tested. They had two studies in the field: one in which the cassava were planted in black plastic bags, and a second later one in which the cassava were planted directly in the field, with uninnoculated cassava as a barrier. Each study would take 11 months—the full time for a cassava to reach maturity.
The first plants in the plastic bags looked a bit sickly; they’d be harvested in October. The second experiment with the plants directly in the ground were flourishing. Those would be harvested the following spring.
Rodriguez is generally the positive one of the pair, sure that they can find a way to work through all challenges. Sanders tends to be more cautious, more pessimistic. “In Switzerland,” he joked, “we think of every single problem that could happen, and people here in Colombia are extremely optimistic—‘No worry! It will work!’” Rodriguez laughed in response. But things were looking good. Both scientists were pleased—even excited—about what they’d seen. Rodriguez’s optimism appeared justified.
Her sunny outlook was tested only a few weeks later. The skies of the llanos, often thick and lazy with morning drizzle, turned dark. The clouds unleashed a month’s worth of merciless rain in only 48 hours. Water swept down over the cassava. When the rains finally faded, plant matter was clogging most of the field drains. Liquid mirrors pooled across the research field. Some of the plants, their roots surrounded by water and gasping for oxygen, listed to the side.
Ceballos, the PhD student in charge of the project, heard the news first. She panicked and ran to Rodriguez to tell her what had happened. Rodriguez panicked as well, thinking, “What are we going to do?” But she quickly regrouped. “We need data,” she told Ceballos, and then called Sanders.

^ Unearthing cassava roots
After a few days, students from Utopia who were dispatched to check on the fields sent back photos. Variation 1, with the older plants trapped in plastic bags, was fine. In the second one with healthier plants, the team received an incredible turn of luck. True, many of the plants were destroyed. But almost none of them had been coated with the fungi. Instead, almost all the dead cassava were just border plants.
Sanders was relieved. “It would have been a disaster for us,” he says, if the plants had died. It would have set the project back at least a year—and the team’s funding was due to end in the summer of 2014.
Three months later, in October, it was time to harvest the plants in the plastic bags. Ceballos headed back to Utopia. Each day for a week, she and another graduate student worked with students, crouching down and cutting open the thick black plastic. They shoved aside the soil that clung, damp, to the roots. The cassava poked out, some thicker than others, all with pale, purplish skin, smooth and wet, peeking through the dirt. Their flesh was bright white and oozed milky droplets.

^ Utopia students weigh cassava roots in the field.
The team uncovered more than a thousand roots. All were quickly weighed at Utopia. Then Ceballos hauled the best, least damaged representatives of each cassava plant back to Bogotá, nearly 800 pounds of food. She stored them in a cavernous new freezer the lab bought specifically for this purpose. Over the next few months, she tested each plant’s dry weight and evaluated its fibrousness, starch content, acid content, and other variables that attest to the overall quality.
Sanders didn’t have high hopes for the first harvest. After all, the crops didn’t look nearly as healthy as the cassava planted straight in the field. But the results thus far have surprised—and delighted—him. The data hasn’t been published in a scientific journal yet, but, he says, “We have actually seen huge differences in the weight of the cassava roots—much larger differences than seen in the rice experiment. We thought it would work but not to such an extent.”
Into the Mainstream
Rusty Rodriguez’s approach is proving successful, too. In 2014, his company is releasing two products, one for rice and one for corn, and he plans to have additional products for a wider variety of crops available by 2015. Based on his company’s field research, test plants are able to tolerate more stress from swings in temperature or water availability, and they can defend themselves more effectively against pests. He says his team is now looking at helping farmers decrease the amount of fertilizer they use by employing the fungi. They’re also publishing scientific studies on their research.
The major agricultural seed and chemical companies are taking notice. In the fall of 2013, Monsanto paid the Danish company Novozymes $300 million to form a partnership called the BioAg Alliance. Novozymes creates what they call “microbial yield and fertilizer enhancers,” among other products in a variety of sectors. The partnership strengthens Monsanto’s role in what they term “sustainable microbial technology.”
The rest of the field seems to be following suit. The trade journal Agrow: World Crop Protection News, wrote that the biopesticide sector was finally no longer “fringe” in April of 2012, and by 2013 proclaimed that it is now an “intrinsic part of the crop protection industry.” In 2012, Bayer bought the small biopesticide company AgraQuest. Syngenta bought Pasteuria Bioscience, and also has an exclusive international deal to sell a Bacillus-based biofungicide. The FDA is testing the spraying of bacteria on tomatoes that can destroy the human-harming salmonella and prevent other forms of contamination.
There are plenty of concerns in the field of applied microbes for agriculture. One is whether any product that is successful on one farm will be equally successful on another. Then there’s the concern about releasing microbes into new environments, which means that regulatory agencies are demanding extensive environmental tests before certifying new products.
The Colombia team is sensitive to this, and they’re studying the existing microbial ecosystems in the presence of the new fungi. They’ve also sent a grad student into the Amazon to collect fungi from wild versions of cassava, fungi that have co-evolved with the cassava for thousands of years, in hopes that they can isolate, grow, and breed these cassava-loving fungi as well.

^ Thin filaments of mycorrhizal fungi form a dense network between roots.
Sanders has an ambitious, seemingly quixotic goal that he figures could be completed in 15 years, maybe 20. He wants to breed enough genetically distinct lines of fungi and try them out with enough crops in enough different environments so that researchers can create what’s called an “association map.” He would start by characterizing the genetics of the fungus and then map them against the crops and the environment. By peering deeply enough into the genetic code, he hopes we can catch a glimpse of which genes make quinoa grow better in Peru, for example. That way scientists could breed a new species of fungus and know in advance which crop it would improve without having to undertake years of trials.
It seems nearly impossible to do enough studies, with enough crops, in enough farmland around the world to generate such a map. Genetic solutions also frequently seem to dance out of reach. Sanders insists, though, that big, crazy scientific goals in agriculture are crucial. “As one of the senior people in the Food and Agriculture Organization of the United Nations said to me, ‘If scientists don’t do that, then we are in trouble in the future.’ I believe he is right.”
Sanders and Rodriguez are now setting up studies in Africa, where farmers, like many in Colombia, can find it difficult to pay for fertilizers and suffer from low yields. Cassava is also one of the top crops there. The team has formed partnerships with local research centers to test varieties of fungi on cassava crops in African soil. They’re hoping the research will begin soon, but they’re still searching for funding.
The scientists believe they’re on their way to achieving their goal of helping farmers grow more food, sustainably. Says Sanders, “We really have to be working extremely hard now to produce the technology that’s going to be used in 10, 15, 20 years’ time. Even if we have something that’s good now, we don’t stop. We have to go for something that’s much better.”
From PBS